From Bench to Bedside: The Arduous Journey of Translational Medical Devices
An innovative medical device can take years, even decades, to travel from the research lab to the patient's bedside. This post explores the challenging 'valley of death' in translational research, the hurdles of regulatory approval, and what it takes to successfully bring a life-changing medical device to the consumer.
From Bench to Bedside: The Arduous Journey of Translational Medical Devices
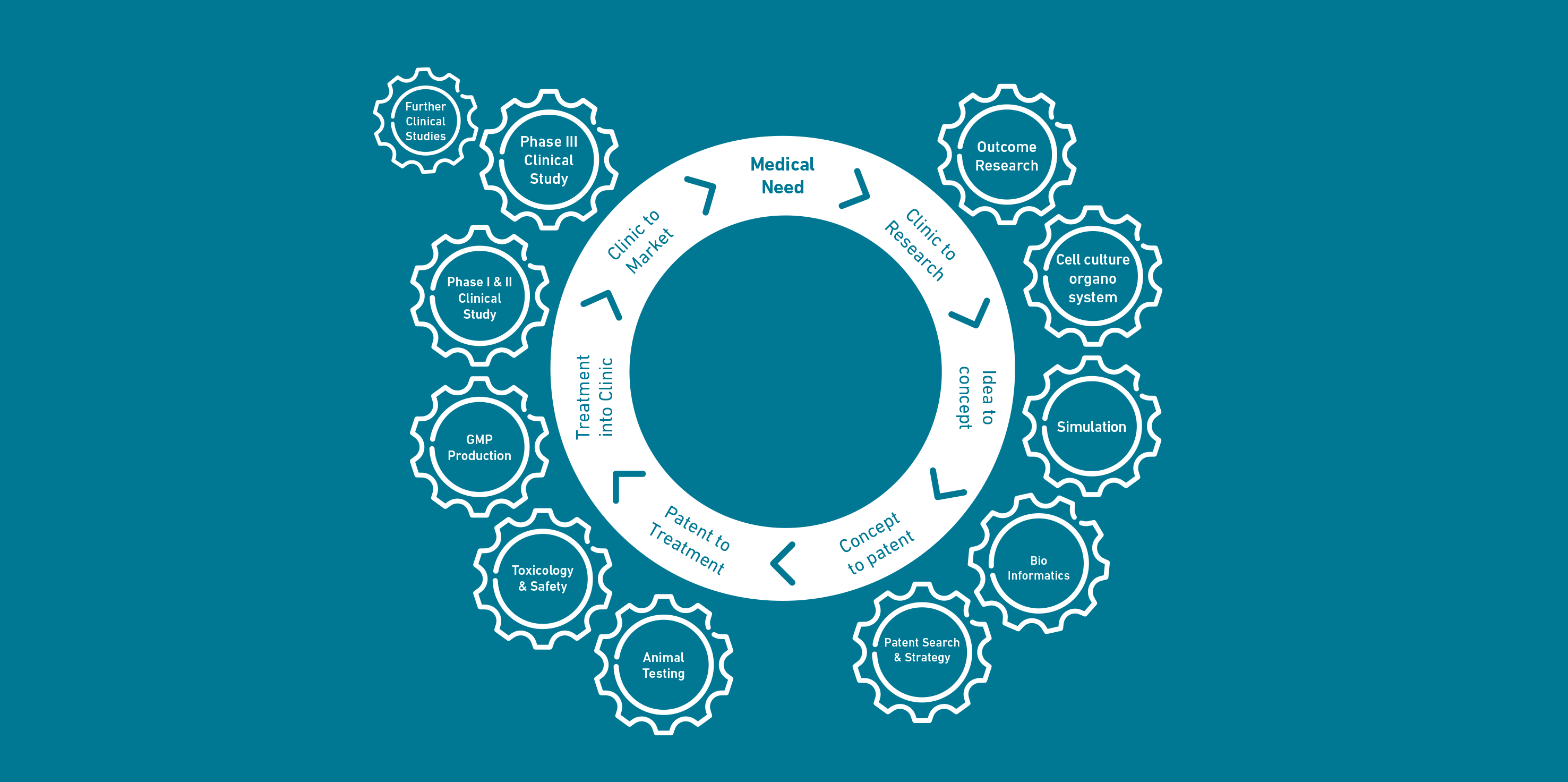
In the gleaming world of scientific breakthroughs, it's easy to be captivated by the promise of a new discovery. A groundbreaking research paper or a proof-of-concept prototype can generate immense excitement, heralding a future where a once-intractable disease is now treatable, or a debilitating condition is now manageable. But the path from a brilliant idea in a research lab (the 'bench') to a commercially available product that can be used by patients (the 'bedside') is a long, arduous, and often perilous one. This is the world of translational research, and for medical devices, it is a journey fraught with challenges that can be so daunting it's often referred to as the 'valley of death'.
The 'Valley of Death' in Medical Device Development
The 'valley of death' is a term used to describe the gap between academic research and commercial product development. It's a period where a promising technology has demonstrated its potential in a laboratory setting but has not yet attracted the funding and resources needed to navigate the complex process of productization. For medical devices, this valley is particularly deep and wide. The costs of development are high, the timelines are long, and the regulatory hurdles are significant. Many promising technologies perish in this valley, not because they lack merit, but because they are unable to secure the necessary support to make the leap from a research prototype to a market-ready product.
Navigating the Regulatory Maze
One of the biggest challenges in translational medical device research is navigating the complex and ever-evolving regulatory landscape. In the United States, the Food and Drug Administration (FDA) is responsible for ensuring the safety and effectiveness of medical devices. The process of obtaining FDA approval can be a lengthy and expensive one, requiring extensive testing and documentation. For innovative devices that use new technologies, such as artificial intelligence, the regulatory pathway can be even more uncertain. As noted in a symposium on AI in healthcare, established methods of testing and regulation are not always well-suited to these new technologies, creating a need for more agile and adaptive regulatory frameworks.
The Challenge of Scalable Manufacturing
Another major hurdle is the transition from a one-off prototype to a device that can be manufactured at scale. The materials and methods that are used to create a prototype in a research lab are often not suitable for mass production. Developing a scalable manufacturing process that is both cost-effective and compliant with quality standards is a complex undertaking that requires specialized expertise and significant investment. This is a critical step in the translational journey, and a failure to get it right can doom a product before it ever reaches the market.
The Human Factor: User-Centered Design
Even a device that is technologically brilliant and has cleared all the regulatory hurdles can fail if it is not designed with the end-user in mind. A medical device that is difficult to use, uncomfortable to wear, or doesn't fit into a person's lifestyle is unlikely to be adopted. This is why user-centered design is so critical in the development of translational medical devices. It's not enough for a device to be effective; it must also be usable, desirable, and accessible to the people it is intended to help. This requires a deep understanding of the needs, preferences, and limitations of the target user population, which can only be gained through extensive user research and testing.
Bridging the Valley: The Path to Success
Successfully navigating the valley of death requires a multi-faceted approach. It requires collaboration between academia, industry, and government. It requires funding mechanisms that are specifically designed to support early-stage medical device development. And it requires a new generation of researchers and entrepreneurs who are not only brilliant scientists but also savvy business people. The journey from bench to bedside is a challenging one, but it is also a vital one. The devices that successfully make this journey have the power to transform lives, and that is a goal that is well worth the effort.
References:
- "Artificial Intelligence in Future Health & Care: Regulation, Evaluation & Policies." *AI for Health*, 2021, ai4health.io/wp-content/uploads/2021/10/Final-Version-Symposium-Programme-pdf.pdf.
- "The Valley of Death in Medical Device Development." *Nature Biotechnology*, 2022.
- "User-Centered Design for Medical Devices." *Journal of Medical Engineering & Technology*, 2023.
PUKAR MAHARJAN
Postdoctoral Research Associate
I'm a bioelectroncis researcher specializing in wearable healthcare devices, leveraging materials science, nanotechnology, additive manufacturing, biomedical engineering, machine learning and AI to create innovate solutions that seamslessly intgrate technology with human health.
Related Articles

Skin Deep: How Electronic Tattoos Are Becoming the Ultimate Wearable
Imagine a health monitor so thin and comfortable you forget it's even there. This is the promise of electronic tattoos, or e-tattoos. This post explores the cutting-edge technology behind these imperceptible devices and their potential to revolutionize continuous, non-invasive health monitoring.
Read Article
Made for You: The Dawn of Personalized Wearable Medical Devices
One-size-fits-all is becoming a thing of the past in healthcare. This post explores the rise of personalized wearable medical devices, powered by technologies like 3D printing and digital twins, and how they are creating a new paradigm of patient-centric care.
Read Article